Matter under Extreme Conditions, and Dynamics of Physical and Physicochemical Processes
Description
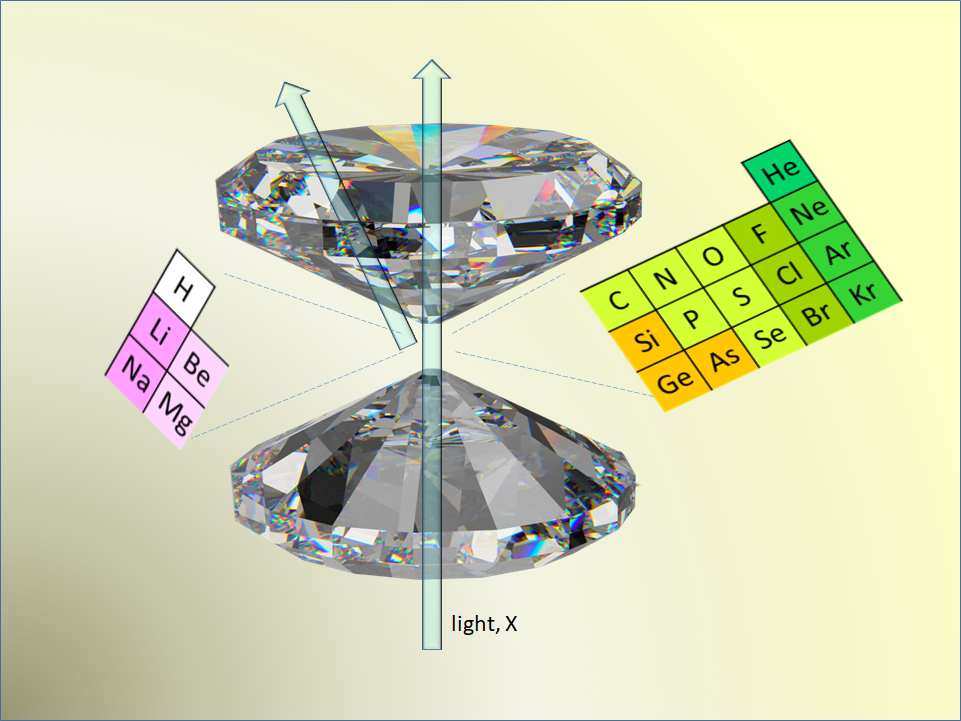
The investigation of MATTER AT EXTREME PRESSURES, up to several millions of atmospheres, and temperatures of 10-6000 K, severely challenges our traditional view of the periodic table of elements, along with the high-school partition of solids into four classes: covalent, metallic, ionic, and molecular solids. For instance, insulating, molecular solids can be transformed into metals and even superconductors, and vice versa. Novel compounds are then obtained, which are superconductors at nearly room temperature, and the “Holy Graal” in this contest is the possible achievement of superconducting hydrogen. High pressures are obtained by squeezing matter between two opposed diamond anvils, in the diamond anvil cell. This cell is coupled to materials characterization techniques such as optical spectroscopy, and synchrotron X-ray diffraction and spectroscopies. Our main fields of investigation are the experimental study of the phase diagram of simple molecular substances and of simple elemental systems, in the solid, liquid or supercritical fluid state, and the chemical synthesis of novel exotic materials often recoverable at ambient conditions.
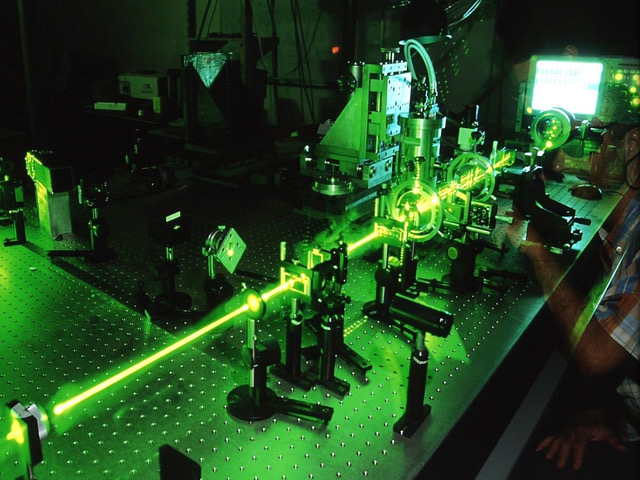
The experimental research on the DYNAMICS OF LIQUIDS AND SOFT MATTER focuses on studying materials with intermediate properties between the liquid and solid states (liquids, glasses, liquid crystals, polymers, colloids, gels, membranes, biological solutions in water, etc.). These systems, despite their variety, share an important common physical feature: molecules self-organize over mesoscopic spatial lengths (nm to micron) resulting in coherent ordered structures and dynamics. Structure and dynamics of soft matter are studied with nonlinear time-resolved laser spectroscopy by exciting the sample in an impulsive manner. Thus, it is possible to follow the response of the sample on a very large time window, from femtoseconds to milliseconds, and study properties such as: molecular vibrations, structural-rotational relaxation, elastic-acoustic propagation, and thermal diffusion depending on the used experiments. Experimental setups: (i) optical Kerr effect experiment (HD-OKE), (ii) transient grating experiment (HD-TG), (iii) THz time domain spectroscopy (THz-TDS), and (iv) photon correlation imaging (PCI).
The research on the DYNAMICS OF PHYSICOCHEMICAL PROCESSES focuses on the development and application of 1D and 2D non-linear pump-probe spectroscopic techniques in both the visible and the infrared region. Their use allows us to collect information about the electronic and structural relaxation processes occurring in the physical systems under investigations. The techniques in play here are: (i) transient absorption spectroscopy, which tracks the temporal evolution of the excited state of a sample after excitation with a short laser pulse, (ii) 2D-electronic spectroscopy, which monitors the temporal evolution of the couplings between different electronic transitions and investigates phenomena such as spectral diffusion and line broadening, (iii) time resolved IR spectroscopy, which is informative about molecular structural changes that occur following photoexcitation (both in the visible and in the IR), (iv) 2D IR spectroscopy, which studies the structural changes on time scales of the picoseconds and the coupling between vibrational modes, and (v) 2D transient IR spectroscopy 2D, which acquires differential 2D IR maps between an excited electronic state and the ground state thanks to a visible pulse sent previously to the sample.
INO Staff
Gorelli Federico AiaceIagatti AlessandroRiboli FrancescoSantoro Mario (Contact Person)Taschin Andrea
Personnel not belonging to INO
Agati Milo (LENS)
Bartolini Paolo (LENS)
Berni Selene (LENS)
Bini Roberto (LENS)
Boschetti Alice (LENS)
Bussotti Laura (LENS)
Caminiti Luigi (LENS)
Catalini Sara (LENS)
Ceppatelli Matteo (ICCOM-CNR)
Cinfrignini Pamela (LENS)
Di Donato Mariangela (ICCOM-CNR)
Doria Sandra (ICCOM-CNR)
Fanetti Samuele (ICCOM-CNR)
Foggi Paolo (LENS)
Lapini Andrea (LENS)
Romi Sebastiano (LENS)
Torre Renato (LENS)
Scelta Demetrio (ICCOM-CNR)
Venturi Sara (LENS)
