HOT PAPER in Angewandte Chemie: Your greatest shade of blue
May 17, 2018
Condensate formation and evolution
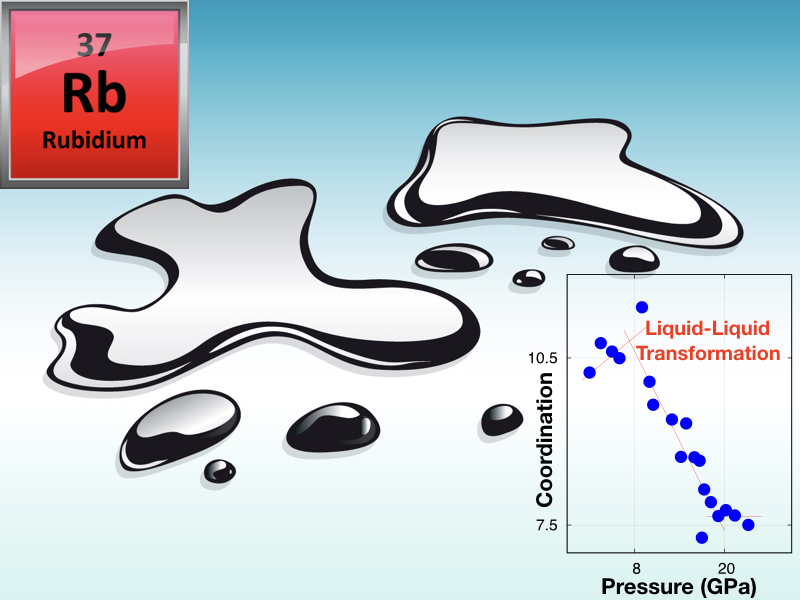
June 12, 2018INO-CNR researchers working on Matter and Materials at Extreme Conditions (Federico Gorelli and Mario Santoro) led a recent challenging study on an archetypal simple metal: Rubidium, at high pressure and high temperature conditions. These researchers, together with colleagues of IIT (Simone De Panfilis) and of the European Synchrotron Radiation Facility, ESRF-Grenoble, found that melted Rubidium undergoes a structural transformation between two distinct liquid forms or phases, at about 75000 atm. and 300 °C. Indeed, liquid-liquid transformations are not common and, consequently, rather surprising, within a traditional view of phase diagrams of pure substances. Common phase transitions for these systems are instead the vapour-liquid, liquid-solid, and vapour-solid phase transitions, whereas this scenario is strongly modified at extreme conditions. In fact, high pressure leads to a plethora of astonishing solid-solid phase transitions along with the here mentioned, more exotic liquid-liquid transition. Demanding synchrotron X-ray diffraction measurements in diamond anvil cells show that while ambient pressure melted Rb is a text-book simple liquid with almost the highest possible atomic packing, the high pressure liquid phase of this element is made of a remarkably more complex and open atomic structure, namely a structure with an increased number of voids. This transformation is traced back to the metallic Rb-Rb bond turning into a bond more similar to those of covalent materials, a modification that may ultimately lead to the metallic-insulator transition already observed in Lithium and Sodium The pressure driven simple-to-complex transformation in liquid Rubidium is likely to be a common feature of all alkali metals, and the discovery of this phenomenon opens a new scenario to the fundamental investigation of liquid matter with potential applications to metallic alloys. The original paper is now published on J. Phys. Chem. Letters (https://pubs.acs.org/articlesonrequest/AOR-d2mSWZNJmqrF25zpvSdD DOI: 10.1021/acs.jpclett.8b01094).